You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Chemical disinfectants serve a very useful purpose in infection control, because many operatory surfaces become routinely coated with saliva, blood, exudate, and other debris, and these surfaces require cleaning with appropriate disinfection when it is not feasible to use disposable covers. Over the years, a number of formulations and technologies have become available, and the area of environmental asepsis continues to be emphasized in Centers for Disease Control and Prevention (CDC) and American Dental Association (ADA) infection control recommendations.1-7
Dental health care workers (DHCW) are faced with many product choices, and unfortunately they sometimes come upon information that can be confusing, thereby making purchasing decisions difficult. Despite extensive literature on this subject, many of the common questions asked by clinicians and their clinical personnel center around what type and/or brand of disinfectant or disposable cover to purchase.
In an effort to address representative concerns, the following discussion will use a question-and-answer format to review, among other issues, basic principles of environmental asepsis, classification and regulation of commercially available products, and product use and misuse.
Where do environmental surfaces and disinfection fit into the classification scheme for contaminated items?
A standard system of classification for chemical sterilants and disinfectants was originally proposed by Spaulding in 1972,8 with a subsequent updated modification in 1991.9 Patient care items and equipment are classified into one of three categories: critical, semicritical, and noncritical, as presented in Table 1.7 Environmental surfaces are included in the noncritical item category and are further subdivided as either housekeeping or clinical-contact surfaces (Table 2) according to the most recent modification of the Spaulding system for classification of disinfectants.10 Each category requires different levels of infection control based on: (1) potential for patient contact; (2) frequency of hand contact; and (3) potential contamination of the surface with body substances or pathogens.
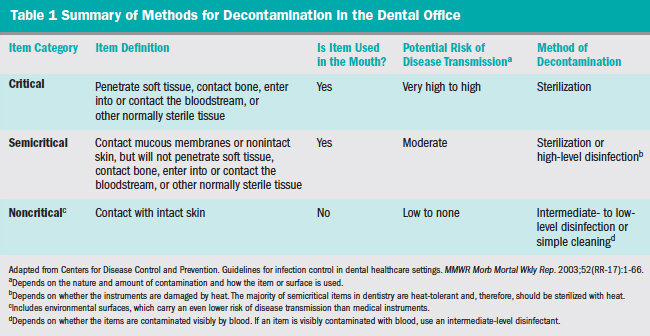
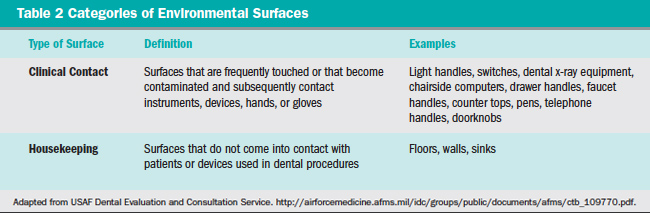
In addition, classes for chemical germicide use are related to their sterilization or disinfection capabilities.7 Classes here include the efficacy against vegetative bacteria, tubercle bacilli, fungal spores, lipid and nonlipid containing viruses, and bacterial endospores.
Why does the Environmental Protection Agency (EPA) require the destruction of Mycobacterium tuberculosis (ie, a tuberculocidal claim) as the benchmark for intermediate-level disinfectants?
M tuberculosis is a highly resistant, vegetative, acid-fast bacillus that is used as the resistance standard for disinfectants because of its innate resistance to chemical germicides. It is therefore used as a “marker” bacterium, and not because of any risk of contracting tuberculosis from a contaminated counter top or light handle. Killing M tuberculosis also destroys other pathogens of occupational significance, including hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), herpes simplex viruses, influenza viruses, and Staphylococcus aureus.
Are there criteria for choosing and appropriately using a surface disinfectant product?
The basic premise to remember is the following phrase: “It’s not necessarily what product you use but how you are using it.” This statement should be combined with the fundamental infection control axiom: clean first. The efficacy of products under consideration should also be compared with published criteria for an ideal agent (Table 3).11 It does not take very long to realize that none of the available products meet all of the criteria. While there is no single best disinfectant, a number of sprays, foams, and wipes have received EPA registration as hospital-level, tuberculocidal disinfectants. The choice often comes down to whether individuals in a practice are going to use a product according to prescribed label directions. Basic concepts like cleaning requirements must be followed to maximize disinfectant efficacy.

What are some of the most common misuses of products used for environmental surface asepsis?
There could probably be a lengthy debate on this topic among any group of respondents. It is beyond the scope of this article to discuss all of the possibilities, so the author will present only a few of the most frequent disinfectant misuses:
1. Forgetting that effective surface disinfection requires initial cleaning of contaminated surfaces to remove the bioburden. The first step in a “spray-wipe-spray” procedure for liquids and a “wipe-discard-wipe” procedure when using disposable wipes is crucial. Cleaning is the removal of visible soil and organic debris, which results in a reduction of the number of microorganisms and removal of organic matter. Cleaning removes the overwhelming majority of biological debris that contains potential pathogens.
2. Excessive aerosolization of sprays causing respiratory, ocular, allergic, and/or dermal irritation problems.
3. Overextending the application area for disposable disinfectant wipes. Most manufacturers of this type of product state on the labels to use multiple wipes to keep treated surfaces wet for the required period. Unfortunately, some personnel may use one or two wipes over a large surface area, not realizing that the antimicrobial agent in the wipe is no longer active or in sufficient concentration. This can result in merely spreading biodebris to other surfaces.
Conclusion
Adherence to a few basic principles and guidelines can assist in the selection and safe use of chemical disinfectants. These include: 1) the product must display an EPA number on its label; 2) the product must be used in strict compliance with printed instructions on the label; and 3) for surface disinfectants to be used on contaminated clinical contact surfaces, the product should state on the label that it is tuberculocidal. These guidelines remain applicable even though these were formulated before the availability of many of the current products.
References
1. American Dental Association Council on Dental Materials and Devices, Council on Dental Therapeutics. Infection control in the dental office. J Am Dent Assoc. 1978;97:673-677.
2. Centers for Disease Control and Prevention. Recommended infection control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1986;35(RR-8):1-12.
3. American Dental Association Councils on Dental Materials, Instruments and Equipment, Dental Practice, and Dental Therapeutics. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1988;116: 241-248.
4. Centers for Disease Control and Prevention. Infection control guidelines for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42 (RR-8):1-12.
5. American Dental Association. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1992;123:1-8.
6. American Dental Association Councils on Scientific Affairs and the Dental Practice. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1996;127:672-680.
7. Centers for Disease Control and Prevention. Guidelines for infection control in dental healthcare settings. MMWR Morb Mortal Wkly Rep. 2003;52(RR-17):1-66.
8. Spaulding EH. Chemical disinfection and antisepsis in the hospital. J Hosp Res. 1972;9:5-31.
9. Favero MS and Bond WW. Sterilization, disinfection and antisepsis in the hospital. In: Manual of Clinical Microbiology. 5th ed. Washington, DC: American Society for Microbiology; 1991:183-200.
10. Favero MS and Bond WW. Chemical disinfection of medical and surgical materials. In: Block SS. Disinfection, Sterilization and Preservation. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:881-917.
11. Molinari JA, Campbell MD, York J. Minimizing potential infection in dental practice. J Mich Dent Assoc. 1982;64:411-416.




